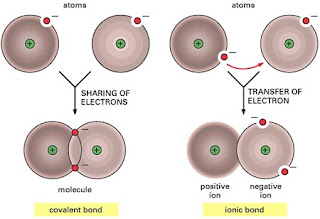
When electrons are shared by two metallic atoms a metallic bond may be formed. In a covalent bond, electrons are shared between two atoms. The electrons that participate in metallic bonds may be shared between any of the metal atoms in the region.
Although the term "metallic bond" is often used in contrast to the term covalent bond, it is preferable to use the term metallic bonding, because this type of bonding is collective in nature and a single "metallic bond" does not exist. Metallic bond is not the only type of chemical bonding a metal can exhibit, even as a simple substance.
No comments:
Post a Comment